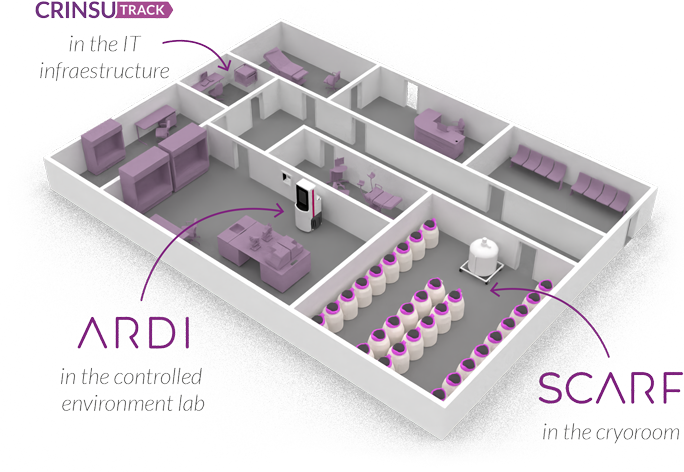

October 25-29 2025
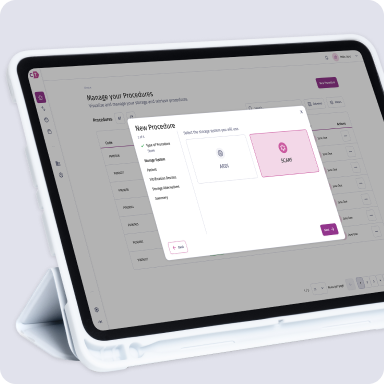
ASRM Scientific Congress & Expo: Introducing SCARF
Helping embryologist to beat the odds
We support embryologists in their mission to help people around the world pursue their deepest family dreams.
Traceability
Tank Status & Alarms
Automation
LN2 Management
Technology
Designed to ensure safety, traceability and efficiency in cryopreservation.
ARDI
The perfect solution for maintaining the cold chain and ensuring the digital custody of cryostored embryos and eggs.
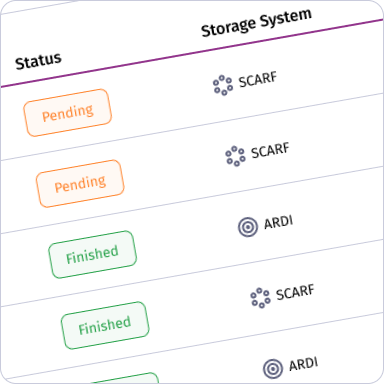
CRINSUTrack
Digital platform that manages cryogenic storage, inventory, and access control.
ARDI's Unique Features
ARDI is an advanced robotic cryogenic storage system built for IVF and other sensitive applications, offering enhanced safety, automation, and space efficiency.
No Manual Refills
Integrates cryocooler technology to eliminate the need for liquid nitrogen refills, reducing handling and improving safety.
In-Liquid Storage
Keeps specimens fully immersed in liquid nitrogen (not vapor), ensuring optimal thermal stability and cold chain security.

Automatic Insertion and Retrieval
Compatible with all straws and witnessing systems, and supports selective “cherry-picking” of embryos based on criteria like PGT.

Compact & High Capacity
Stores samples for 1,000–5,000 patients in a footprint similar to a standard fridge, with no need for auxiliary LN2 tanks.
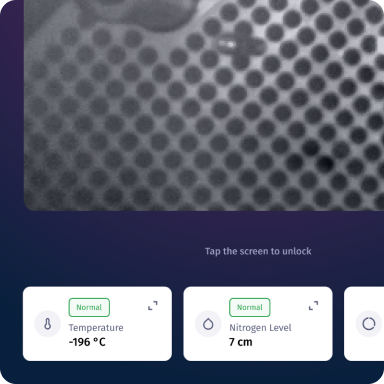
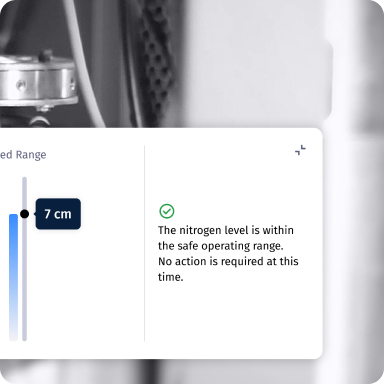
24/7 Monitoring & Visibility
Uses sensors to continuously track temperature and liquid nitrogen levels, while a built-in cryogenic camera allows real-time visual inspection inside the tank.
Advanced Sample Tracking
Uses RFID technology for contactless, accurate identification and full traceability of specimens.
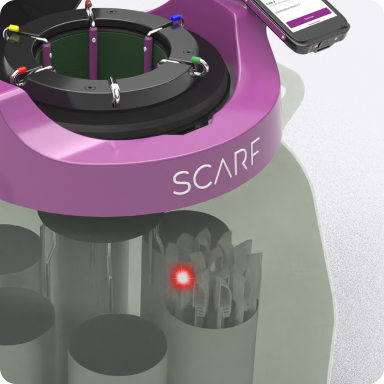
SCARF's Unique Features
SCARF is a smart cryogenic traceability solution designed to improve efficiency, accuracy, and security in lab environments.

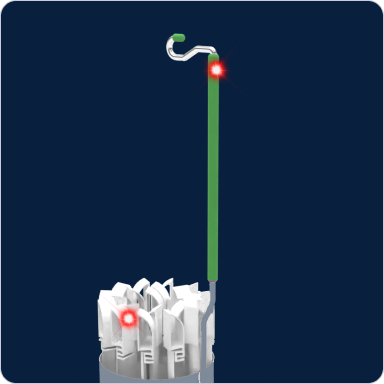
Guided by Light
Uses visual light signals to help users quickly locate specific canisters and sample containers, reducing search time.
Wireless Identification
Each SCARF tag has a unique RFID code, allowing fast, contactless identification of samples without fragile labels.
Access Control
Built-in electronic locking with role-based permissions and automatic logging ensures secure, traceable access to samples.
Digital Inventory
Provides a real-time digital overview of storage status, enhancing planning and eliminating manual checks.

Effortless Setup
Installs easily with no wiring or batteries, integrates seamlessly into lab workflows, and requires minimal maintenance.
Cryogenic-Ready
Engineered to function reliably in extreme liquid nitrogen storage conditions.
CRINSUTrack's Unique Features
CRINSUTrack is a digital platform that manages cryogenic storage, inventory, and access control.

Cryogenic Storage and Inventory Management
Manages cryogenic samples, storage units, and access control, tracking sample locations, tank conditions, and usage history in real time for complete visibility and traceability.
System Integration
Connects seamlessly with ARDI (automated cryotank) and SCARF (smart collar system) to enable end-to-end monitoring and coordination across all cryogenic equipment.
Regulatory Compliance
Ensures adherence to major data protection standards, including GDPR and HIPAA, through secure data handling, audit trails, and detailed access logs.
Flexible Deployment
Operates as a cloud-based platform for centralized management or as an on-premise system for private infrastructures, with scalability across multiple clinics or sites.
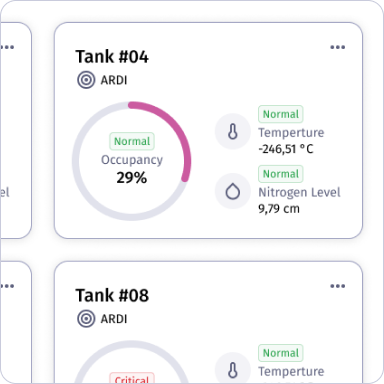
Unified Dashboard
Displays consolidated data from tanks, devices, and facilities, providing real-time monitoring, alerts, and analytics through a single, intuitive interface.
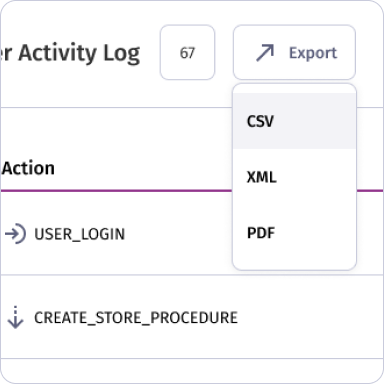
Data Export and Backup
Supports exporting of logs, audit records, and tank inventories in standard formats (CSV, XML, PDF) and enables automated backups for data recovery and reporting.

Contact Us
By submitting this form, you consent to Crinsurance processing your personal data as described in our Terms and Privacy Policy.
After submitting this form, a member of our team will contact you by email.
Company
We are a collective of innovators and thinkers drawn to the most complex challenges in science and technology. Guided by curiosity and integrity, we listen deeply to real-world needs, analyze rigorously, and engineer solutions that bring clarity and safety to the cryogenic frontier.
We turn pioneering concepts into tangible realities that safeguard life at its most delicate stages. Our mission is to make cryogenic preservation smarter, safer, and seamlessly connected—bridging innovation with purpose.
We turn pioneering concepts into tangible realities that safeguard life at its most delicate stages. Our mission is to make cryogenic preservation smarter, safer, and seamlessly connected—bridging innovation with purpose.
We stand at the frontier of the impossible. The era of intelligent cryopreservation is here. The next breakthrough awaits.
Frequently Asked Questions
Everything you need to know.
ARDI
What is ARDI?
ARDI (phonetically derived from “R-D,” Reliquefier Dewar) is an intelligent cryogenic management system developed for fertility and reproductive medicine laboratories. It ensures stable and secure cryostorage conditions by automating liquid nitrogen management and providing complete digital traceability for oocytes and embryos.
What problem does ARDI solve?
Traditional cryostorage management relies heavily on manual operations, which are time-consuming and prone to human error or unnoticed nitrogen losses.
ARDI eliminates these risks by automating both sample handling and reliquefaction.
Its integrated robotic system performs storage and retrieval operations without manual intervention, reducing exposure to ambient conditions and ensuring consistent, error-free handling that safeguards biological integrity and regulatory compliance.
ARDI also eliminates the need for external LN₂ refills by continuously reliquefying gaseous nitrogen within its closed system.
How does ARDI maintain cryogenic safety?
ARDI continuously monitors LN₂ level and internal pressure. When the level approaches predefined thresholds, its integrated LCS (Level Control System) regulates the cryocooler and valves to reliquefy gaseous nitrogen within the closed Dewar.
This maintains temperature and pressure within safe cryogenic ranges, ensuring long-term stability without manual intervention.
The LCS works in conjunction with redundant sensors and automatic safety valves that prevent overpressure or excessive boil-off.
How does the robotic arm assist sample management?
The Place and Retrieval System (PRS) autonomously handles the positioning, storage, and retrieval of ARDI containers and vitrification devices inside the Dewar.
It combines motion control with onboard sensors to ensure precise operation even under cryogenic conditions.
The PRS minimizes operator exposure to cold zones, prevents misalignment or cross-handling errors, and guarantees consistent placement accuracy—critical for maintaining chain of custody and biological integrity.
It is compatible with all vitrification devices (straws and carriers) currently used in fertility laboratories, ensuring seamless integration with existing workflows.
Can ARDI alert the user of abnormal conditions?
Yes. ARDI provides both visual and remote alerts (via LEDs, on-screen messages, and email or mobile notifications) whenever it detects abnormal conditions.
Each event is logged with an exact timestamp and can be reviewed locally or remotely through the connected Crinsutrack platform.
What makes ARDI intelligent?
ARDI integrates local control logic and predictive algorithms that analyze sensor data trends to anticipate anomalies—such as leak formation, insulation degradation, or nitrogen depletion—before they occur.
Combined with remote analytics through Crinsutrack, it transforms raw telemetry into actionable insights for preventive maintenance and operational optimization.
Can ARDI operate without constant human supervision?
Yes. Once configured, ARDI autonomously maintains cryogenic stability and traceability.
Its control systems automatically regulate LN₂ levels, pressure, and temperature, while keeping full event logs.
Personnel are only notified when direct human action is required, minimizing routine workload and human error.
Does ARDI include sample tracking? Is it compatible with SCARF?
It is fully compatible with SCARF through the Crinsutrack software.
Is ARDI compatible with existing clinics?
Yes. ARDI has been designed to fit seamlessly into any fertility clinic. Its original dimensions were developed to be compatible with a standard clinic door size of 900 mm × 2000 mm.
Installation requires no infrastructure modification or external gas lines.
How long does installation take?
Installation typically takes two days, depending on the model and clinic layout.
Once connected to power and the network, ARDI automatically performs self-calibration and verifies sensor integrity.
No external LN₂ or cooling source is required; the system operates as a fully closed loop.
Does ARDI connect to CrinsuTrack or other systems?
ARDI connects directly with Crinsutrack for cloud-based monitoring, audit logging, and access management.
Its open API (REST-based) enables interoperability with third-party LIMS and EMR systems using encrypted TLS communication, ensuring seamless data exchange between platforms.
Does ARDI comply with medical-device safety standards?
ARDI has been designed and verified in accordance with internationally recognized standards for electrical safety, electromagnetic compatibility, software lifecycle, usability, and risk management.
These include IEC 61010-1 for safety, IEC 61326-1 for EMC, IEC 62304 for software, IEC 62366-1 for usability, and ISO 14971 for risk management.
All processes are implemented in accordance with the ISO 13485 quality management system standard.
What happens during a power failure?
In the event of a power outage, ARDI maintains safe cryogenic temperatures for at least 10 days.
Its integrated UPS provides enough backup to safely complete any ongoing retrieval or storage operations, maintain telemetry and audit traceability for up to three hours, and automatically secure the system by closing LN₂ valves.
Once power is restored, ARDI automatically resumes normal reliquefaction and monitoring operations.
How does ARDI ensure data security?
Minimal. Preventive maMinimal. Preventive maintenance is required only twice per year.
An annual inspection and calibration—performed by CRINSURANCE or authorized service partners—ensures continued reliability, accuracy, and regulatory compliance.intenance is required only twice per year.
An annual inspection and calibration—performed by CRINSURANCE or authorized service partners—ensures continued reliability, accuracy, and regulatory compliance.
Does ARDI require regular maintenance?
Minimal. Preventive maintenance is required only twice per year.
An annual inspection and calibration, performed by CRINSURANCE or authorized service partners, ensures continued reliability, accuracy, and regulatory compliance.
How is ARDI verified or validated?
Each ARDI unit undergoes full verification against its system, functional, and safety requirements (NFR, SyR, SS) before delivery.
All units are individually tested, calibrated, and documented with conformity and calibration reports.
ARDI has also been designed and validated under strict international standards, including IEC 61010-1, IEC 61010-2-10, IEC 61010-2-11, IEC 61326-1, IEC 61326-2-6, and CISPR 11, as well as FDA interoperability guidance, 47 CFR Part 15 for RF compliance, and ISTA 2A for packaging validation.
Is technical training provided?
Yes. Comprehensive training materials, detailed user manuals, and remote onboarding sessions are included with every unit.
On-site setup, operation, and validation training can be arranged for clinics or multi-unit installations to ensure optimal system use and maintenance.
What ARDI configurations are available?
ARDI is available in three configurations, Standard (≈5000 samples) and Advanced (≈9500 samples), each sharing the same control platform and cryogenic management architecture, differing only in storage capacity.
How many ARDI units do I need?
Usually, one ARDI per laboratory is enough. It manages the active inventory, the samples frequently inserted, retrieved, or relocated, ensuring automation, traceability, and safety where the lab interacts most.
Clinics with very large or specialized programs may use multiple ARDIs to segment operations or increase capacity.
Can I request a pilot or demo unit?
Demo units are not shipped, but full ARDI systems are available for hands-on evaluation at CRINSURANCE facilities. Visits can be scheduled to test and experience ARDI in operation.
Where can I purchase ARDI?
Go to Contact Us section on this webpage.
SCARF
What is SCARF?
SCARF (Smart Cryogenic Assisted by Radio Frequency) is a smart collar system that mounts onto cryogenic dewars, providing wireless identification, access control, and assisted localization through light-guided technology. It transforms traditional cryostorage into an intelligent, connected environment.
What problem does SCARF solve?
Manual cryostorage management is time-consuming, error-prone, and often lacks traceability. SCARF eliminates uncertainty by offering real-time visibility of stored materials, guided retrieval through intelligent lighting, and secure access logging—reducing risks, time, and liability.
How does SCARF identify samples?
Each canister or sample holder is equipped with RFID tags that communicate wirelessly with the SCARF collar. The system recognizes and locates each item without requiring physical handling or exposure to ambient conditions.
What is the light guidance system?
SCARF’s pick-to-light technology visually guides users with LED sequences to the exact canister or position where the desired item is located, simplifying retrieval and reducing human error.
Does SCARF require batteries or wiring?
No. SCARF is powered entirely through its docking connection with the mobile device—no internal batteries or external cables are needed, minimizing maintenance.
Can SCARF work with existing dewars?
Yes. SCARF is designed for quick retrofit installation on standard cryogenic dewars (such as MVE 47/11) without modifications or tools.
What models of dewars are supported?
The preferred host is the MVE 47/11, though SCARF can be adapted to other models upon request.
What happens if there’s a power or system failure?
SCARF includes a secure emergency access mode with tamperproof detection. Even in offline conditions, access is logged and synchronized when power is restored.
How long does installation take?
SCARF is ready to use from day one. Most users can install it in minutes—mount the collar, dock the mobile device, and the system auto-detects the hardware.
Is software required?
Yes. SCARF integrates with CrinsuTrack, CRINSURANCE’s digital inventory and access management software. It provides a full dashboard of tank capacity, sample location, and user activity.
Does SCARF affect cryogenic performance or temperature?
No. SCARF operates externally, without interfering with liquid nitrogen levels or thermal insulation. All components are engineered for cryogenic environments.
What maintenance does SCARF require?
None. SCARF has no moving parts, no batteries, and no exposed electronics. Routine visual inspection is sufficient.
How can I get technical support or training?
Training, setup videos, and user manuals are available through the CRINSURANCE support portal. On-site or remote assistance can be arranged for multi-unit installations.
How many SCARFs do I need for my lab?
Typically, one SCARF per dewar. Each unit manages all canisters within its tank and connects to a shared inventory system.
Can I start with a pilot unit?
Yes. Starter kits are available for evaluation, including a SCARF collar, RFID canister tags, and access to the digital dashboard.
Where can I buy SCARF?
Go to Contact Us section on this webpage.
CRINSUTrack
What is CRINSUTrack?
CRINSUTrack is a digital platform that manages cryogenic storage, inventory, and access control. It integrates seamlessly with ARDI (the automated cryotank) and SCARF (the smart collar system) to provide complete traceability of samples and cryogenic assets.
What problems does CRINSUTrack solve?
It eliminates manual recordkeeping, reduces identification errors, and provides real-time visibility of cryogenic assets. CRINSUTrack ensures every action—from freezing to retrieval—is traceable, authorized, and recorded.
Is CRINSUTrack cloud or on-premise?
CRINSUTrack can operate in both modes:
- Cloud-based for centralized management across multiple sites.
- On-premise for clinics preferring offline or private operation.
How does CRINSUTrack connect to ARDI?
When linked to ARDI, CRINSUTrack monitors the cryogenic environment, logs tank operations, and records user access. It serves as the ARDI’s control and audit interface.
How does it integrate with SCARF?
With SCARF, CRINSUTrack identifies all tagged canisters, enables pick-to-light retrieval, logs user access, and maintains an updated inventory of each dewar in real time.
Can both ARDI and SCARF be managed in the same dashboard?
Yes. The same CRINSUTrack interface manages multiple devices, clinics, and tanks. Each tank’s data (capacity, samples, access log, and location) appears in unified views.
Can CRINSUTrack manage cryostorage not equipped with ARDI or SCARF?
Yes. It also functions as a stand-alone digital logbook for conventional tanks. You can manually record storage positions and track movements without connected hardware.
Is CRINSUTrack compliant with data privacy regulations (e.g., GDPR, HIPAA)?
Yes. CRINSUTrack is designed to support compliance with major data protection frameworks.
Can data be exported or backed up?
Yes. Administrators can export logs, audit trails, and tank inventories in standard formats (CSV, XML, PDF) for backups or reporting.
How is CRINSUTrack licensed?
It is licensed per clinic, with plans that scale according to the number of tanks, users, and connected devices.
What’s included in the license fee?
- Software access and updates
- Technical support according to the chosen plan
- Data hosting and backup (for cloud users)
- Integration with ARDI and SCARF hardware
Can I try CRINSUTrack before purchasing?
Yes. A demo environment can be provided for evaluation, showing live simulations of ARDI and SCARF integration.

Headquarters
44 Street No. 793, La Plata, Buenos Aires 1900, Argentina.
Manufacturing
Yongyi 6th Road No. 5, Yongxing Industrial Zone, Henglan, Zhongshan 528478, China.




